- Home
- Ricoh's Technology
- Human drug efficacy and toxicity evaluation plate
Human drug efficacy and toxicity evaluation plate
Background
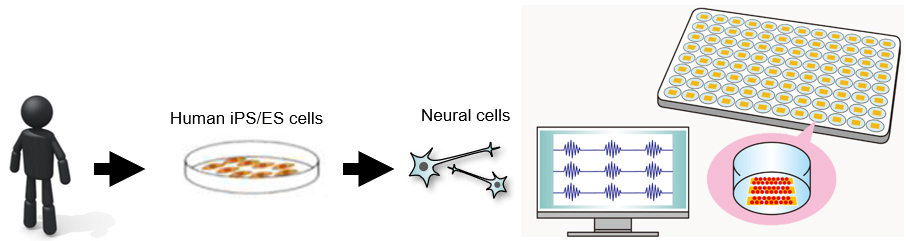
In the development of new drugs, animal models are generally used to confirm the drug's effcacy and toxicity. However, since human cells differ from other animals, toxicity against humans may become evident in the clinical trial stage. To solve the problems of neural systems that are greatly affected by the specific differences between humans and animals, the development of evaluation systems using human iPS cells is being carried out. A representative technique is Multi-Electrode Array (MEA). In MEA assay, iPS cell-derived nerve cells are seeded and cultured on multiple electrodes indwelled on the bottom of each well. Production of nerve cell plate by the users themselves involves tremendous labor, cost, and risks, including cell culture know-how, a prolonged culture period, and problems such as agglutination and stripping off cells. Nerve cell plates are being sought that require no cultivation and can be used quickly.
- iPS cells:
- Induced pluripotent stem cells. This refers to pluripotent stem cells that were artificially produced by culturing different cells
Solution
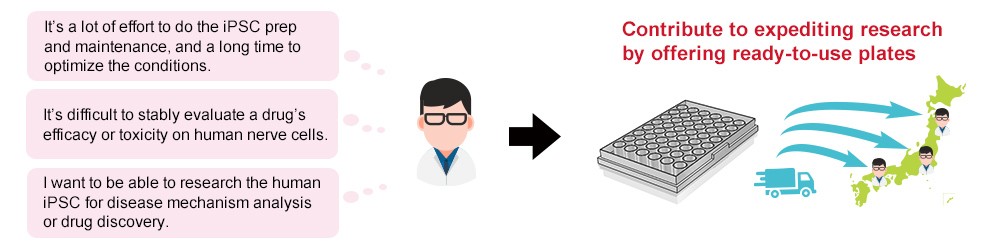
The human neural drug efficacy and toxicity evaluation plate that Ricoh has developed dramatically improved cell adhesion strength by applying a material process technology that the company refined through its bioprinting development operations, thereby solving problems seen during the long-term cultivation period, such as agglutination and cells stripping-off, and enhancing plate quality. By offering human neural drug efficacy and toxicity evaluation plates to new drug researchers in a ready-to-use state, we contribute to shortening the research period, reducing the risks of cultivations failing, and accelerating drug discovery research.
Technical Highlights
In the human neural drug efficacy and toxicity evaluation plate manufacturing process currently developed, we use iPS cells differentiated into nerve cells via Elixirgen Scientific's high-speed differentiation-inducing technology. Inking these cells and combining them with materials and process technologies, cultivated with Ricoh's bioprinting and ink development operations, has made it possible to produce stable quality plates.
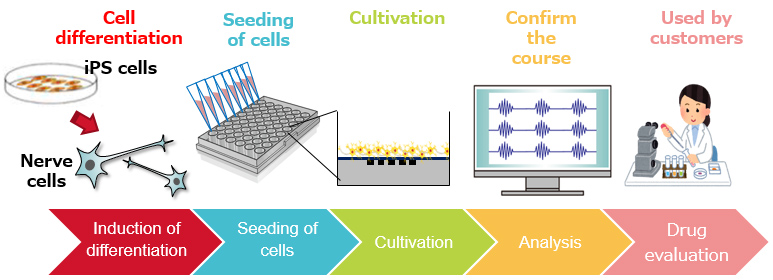
1. Differentiating iPS cells at high speed into human nerve cells
Ricoh's joint business partner, Elixirgen Scientific, developed technology to differentiate and induce iPS cells in a short period and at a high-efficiency level into a variety of cells. Nerve cells that have been differentiated with this technology are also being used for these plates. Cells produced using this technology have functions close to those of mature cells and are expected to solve the problems of differences in species between humans and animals in drug efficacy and toxicity evaluations.
2. High cell adhesive power that makes use of bioprinting technology
MEA plates seeded with iPS cell-derived nerve cells are beginning to be used as an evaluation system in drug discovery research. However, their production requires long-term cultivation and specific know-how. Ricoh used its bioprinting technologies and ink development materials technology to enhance cell adhesive properties and developed human neural drug efficacy and toxicity evaluation plates with minimal variations. Since the produced plates can also be transported over a long distance, it became possible to offer high-quality plates to all domestic researchers that can be immediately used for evaluation as soon as they reach the users.
Ricoh's vision
Healthcare is an area where there is an urgent need to address an aging society's issues, reduce medical expenses, and resolve differences in regional medical quality levels. Ricoh has positioned this sector to solve social problems and challenges and decided to enter it in 2016 as a new business field. We will employ this technology and aim to contribute to medical drug discovery research.
Sorted by : field “Bioprinting” | product type “Healthcare”